GRAPHIC BY NICK ORABOVIC
In industry classes, at trade shows, in articles and in product manuals, the word “electrolysis” comes up in a wide range of contexts. Many service technicians know that it’s the process electrolytic chlorine generators (ECGs) use to make chlorine from salt water. But others also warn that it can contribute to certain types of metal corrosion.
What exactly is electrolysis — and how can one process be involved in such seemingly unconnected chemical reactions?
Experts in pool chemistry have the answers. Here, they share their explanations of electrolysis, from its effects on pool equipment all the way down to its contributions on a molecular level.
A thorough grasp of electrolysis is far more than just “book knowledge” — it enables more accurate diagnoses of many pool problems that other service people might incorrectly attribute to water chemistry.
Understanding its nature and effects will also contribute to better pool maintenance overall — not only for salt pools, but for any swimming setup.
Chemistry
Proper comprehension of the effects of electrolysis must begin with an awareness of how the process works at the chemical level. Professionals throughout the pool industry hold some diverse opinions about what, precisely, electrolysis is — some say it involves metals, while others mention water or salt.
According to chemists, electrolysis is simply the process of using electric current to drive a chemical reaction that wouldn’t occur spontaneously. This can be intentional or accidental, and it requires just three types of components: A direct current (DC) electrical supply, a pair of electrodes to conduct the electrical current, and an electrolyte — a substance containing mobile free ions (positively or negatively charged atoms) to carry the electric current.
“The electrolysis itself is essentially the transfer of electrons from one electrode into the solution, and then from different components in the solution back into the other electrode,” says Zach Hansen, technical services engineer at BioLab Inc. in Lawrenceville, Ga.
As in any electrical circuit, electrons — the particles responsible for negative electrical charge — need a pathway through which to flow. At one point in this pathway sits the anode, from which electrons flow into the metallic portion of the circuit. At the other end of the circuit’s metallic portion sits the cathode, which receives electrons that have come through the metal from the anode. Meanwhile, in the electrolyte portion of the circuit, negatively charged ions (anions) move toward the anode, while positively charged ions (cations) move away from it and toward the cathode.
What makes an electrolysis circuit unique is the electrolyte — the solution that allows ions (and thus, electrical current) to move around relatively freely. This contrasts with circuits in which the current is restricted solely to the movement of electrons through metallic conductors, such as wires. Certain atoms, molecules or ions in the electrolyte accept the free electrons that flow in from the cathode, while others have their electrons pulled away by the positive charge of the anode — and these changes cause those atoms, molecules or ions to transform into ions, molecules or atoms of another oxidation state.
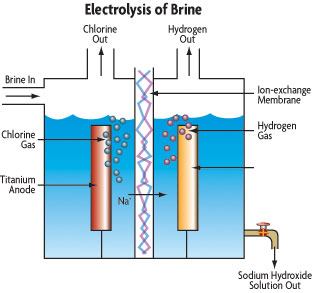
For example, when common salt — known chemically as sodium chloride (NaCl) — dissolves in water, it splits up into two ions — positively charged sodium (Na+) and negatively charged chloride (Cl–). As electric current passes through salt water, electrons are ripped away from the chloride ions and pulled toward the positive charge of the anode. The removal of these electrons converts the chloride ions into neutral chlorine atoms, which are then able to join into molecules of electrically neutral chlorine gas (Cl2) — one form of the commonly mentioned “free available chlorine” that acts as an oxidizer in swimming pools.
Having a clear sense of how the electrolytic process works in general, at the level of individual atoms, will make it much easier to understand how it contributes to such a wide variety of effects up here at the macroscopic level.
Salt chlorination
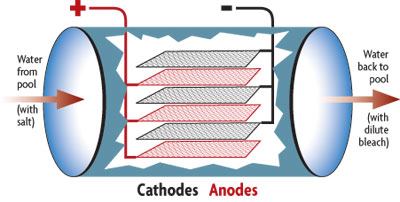
The most common purposeful use of electrolysis in the pool industry is for electrolytic salt chlorination — the creation of free available chlorine from the chloride ions found in salt water. Though the terms “electrolysis” and “salt chlorination” are sometimes used interchangeably, they aren’t actually synonymous — electrolytic salt chlorination is just one application of electrolysis.
As the “Chemistry” section above explained, passing electric current through salt water leads to the conversion of chloride ions into molecules of chlorine gas (Cl2). As this happens, the chlorine molecules diffuse away from the anode and react with water molecules (H2O) to form hypochlorous acid (HClO) or hydrochloric acid (HCl).
But what happens to the positively charged sodium ions (Na+) that are left over? They’re drawn toward the cathode, where they balance the negative charge of the free-floating hydroxide ions (OH–) which are formed by reduction of water molecules at the cathode. The resulting solution near the cathode is sodium hydroxide (NaOH) — a caustic base commonly known as lye. The sodium hydroxide thus formed will quickly react with the chlorine formed at the anode — or with the hydrochloric and hypochlorous acid made by the reaction of chlorine with water — to make sodium hypochlorite (bleach) and sodium chloride (salt). It also reacts with the sodium bicarbonate (“bicarb”) that acts as a pH buffer in the pool’s water to form carbonate — which soon bonds with positively charged calcium ions concentrated around the cathode.
Because of this series of reactions, scale tends to accumulate on the cathode, where it often creates problems for the flow of electric current. Thus, some manufacturers design their chlorine generators to flip the direction of electrical current back and forth on a regular basis — turning the anode into the cathode, and vice versa. This is known as reversing polarity, and it allows the high-pH areas to be bathed in hypochlorous and hydrochloric acid, which help dissolve any scale that’s beginning to accumulate.
Thus, if there’s scale buildup in a chlorine generator — but not in other areas of the pool — there may be problems with the ECG’s electrolytic circuit. “Scale is public enemy number one for the ECG,” says Geoffrey Brown, a developmental scientist at Pristiva Inc. in Overland Park, Kan. “If you get scale, you could get heat buildup inside the cell, and you could ultimately ruin your ECG and have to replace it. And even in the short term, your ECG will have to work harder to make chlorine.”
Because of this potential for long-term damage, it’s important to immediately investigate scale treatments such as antiscalants, acid washes for the plates, or — if applicable — performing or outsourcing an electrical repair to restore the generator’s ability to reverse polarity.
Galvanic corrosion
As electricity flows through water, its influence isn’t always limited to intended chemical reactions. One potentially problematic side effect of electrolysis is galvanic corrosion — the gradual disintegration of one metal that’s in direct metallic contact with another metal while both metals are in contact with the same electrolyte.
The term “dissimilar metals” is commonly brought up as a cause for galvanic corrosion, but the phrase is often misunderstood — galvanic corrosion won’t necessarily happen just because two metals in an electrolyte have different names. Instead, the term refers to the metals’ relative positions on a galvanic corrosion chart, which compares the similarity of metals on an electrochemical basis. Metals toward the “less noble” (anodic) end of the chart are more easily oxidized — and thus, more likely to be corroded — than those toward the “more noble” (cathodic) end. In fact, the word “noble” in reference to metals just means “resistant to oxidation.”
But what exactly is oxidation, and what does it have to do with electrolysis? In general terms, oxidation refers to an interaction between an oxygen (O2) molecule and any other substance. If one type of metal in an electrolytic circuit is more easily oxidized — that is, more prone to react with oxygen — than another metal in contact with the same electrolyte, the flow of electricity will accelerate that process of oxidation, eventually leading to rust or other forms of corrosion.
To put all this in more specific electrochemical terms, oxidation often involves the loss of one or more electrons by a molecule or atom. For example (as indicated on the chart), iron that’s connected to an electrolytic circuit will have a greater tendency to lose one or more of its electrons than copper will. This electron loss will leave room for the oxygen in free-floating hydroxide ions (OH–) to bond with the iron (Fe), forming iron (ferric) oxides such as FeO, Fe3O4 or Fe2O3 — in other words, rust.
Though the presence of applied electric current accelerates oxidation reactions, merely placing two dissimilar metals in an electrolyte is sometimes enough to generate an electric current, as electrons from the less noble (anodic) metal naturally drift away from the electrolyte through the metallic part of the circuit toward the more noble (cathodic) metal. In fact, this is exactly how the earliest batteries generated electricity. Though this process brought some obvious advantages to its users, they also realized that the life of a battery is inherently limited — if the electrochemical difference between the metals is strong enough to generate much electric flow, one of the metals must oxidize and degrade fairly rapidly.
However, this very principle has been adapted for a more helpful purpose in home and pool appliances such as water heaters. In some of these appliances, iron may serve a useful structural purpose, but must be kept sealed off from a water-filled area that would allow it to form an electrolytic circuit with a dissimilar metal, such as a copper pipe nipple. In a case like this, a “sacrificial anode” made from an even less-noble metal — such as zinc — might be installed in the water heater in such a way that it makes direct physical/ electrical contact with the steel of the water tank. Then, if a crack develops in the glass lining of the tank — exposing the steel itself to the water — the sacrificial zinc anode will corrode first, transferring electrons through the point of metal-to-metal contact to the iron, making the iron cathodic. Being negatively charged or cathodic, the steel will not oxidize or corrode until the sacrificial anode is fully spent and the cathodic protection is lost.
Galvanic corrosion can also happen in less predictable circumstances, though — for example, it may take hold in pool environments where dissimilar metals are in contact both with one another and with salt water, which is more electroconductive than pure H2O. In a circuit like this, electrolysis and galvanic corrosion can occur in the absence of any applied electrical current — the electrochemical difference between the metals, and the conductivity of the water, are enough to produce an electric current.
“Galvanic corrosion is an instance where the influence of electrolysis is so often missed, because people don’t recognize it for what it is,” says John Puetz, director of technology at Advantis Technologies Inc. in Alpharetta, Ga. “They can’t understand why this corrosion is happening, because there’s nothing wrong with the water chemistry — but in fact, the chemistry can be perfectly fine and galvanic corrosion can still occur.”
One helpful tip for diagnosing galvanic corrosion is the fact that it often — though not always — happens near a weld or other point of electrical contact between dissimilar metals. “If you see that corrosion is much more severe near such a point of contact than it is farther away from that point of contact, you can be suspicious that galvanic corrosion is involved there,” says Stan Pickens, a senior research associate at PPG Industries in Monroeville, Pa.
In cases like these, one way to prevent galvanic corrosion is simply to break the circuit. “You can greatly slow down the rate of corrosion — or perhaps even stop it entirely — simply by installing a ceramic junction between those two metals,” Puetz says. Other pool professionals recommend PVC pipe fittings. In either case, interrupting the flow of electric current will reduce the rate at which electrons are pulled away from the anodic metal, making it much less likely to oxidize and corrode. Installing a sacrificial anode, as discussed just above, is an alternate means of protecting base metals from corrosion.
The chemistry of salt water can also play a part in galvanic corrosion: As iron oxidizes, some of the ferric oxides described above may form a fairly inert coating on the iron, which can help protect the metal against further corrosion. However, salt water may counter that protective effect. “Chloride in the water tends to destabilize — depassivate — an oxide layer, so that’s one reason that chloride salts tend to make water more corrosive,” Pickens says.
As these examples demonstrate, metals, water, electric current and salt can all interact in a variety of ways to produce a wide range of effects. Thus, a solid grasp of electrolysis makes it much easier to understand why these reactions can lead to results as diverse as chlorine generation and metal corrosion. It’s just one more way in which a little chemical background can vastly improve the care regimen for a pool and its equipment.