When I entered the pool industry in 1973, I recognized the need for accurate and practical pool chemical information. Much of what I heard seemed to be myth and hand-me-down information. So I started writing articles and giving seminars about pool chemistry. Now, some 45 years later, I have written 19 books on swimming pool chemistry and given hundreds of seminars. I am still learning and working to take complex chemistry and reduce it to understandable, useful information for service technicians and retail stores.
About 8 years ago, it occurred to me that pool chemistry is still not as well-understood as it should be in the industry. So I began analyzing the problems that service technicians experience and trying to devise a maintenance method that is logical, scientifically based, yet easy, and that includes only basic pool chemicals — liquid chlorine or bleach, muriatic acid, bicarb, borate and air. I came up with this method after doing some lengthy and in-depth research into cyanuric acid (CYA), chlorine effectiveness in the presence of CYA, and utilizing an old concept of using aeration and turbulence to raise pH without changing alkalinity. While performing this research, I also discovered that liquid chlorine does not raise the pH of pool water.
Last year, I released a new book called Pool Chemistry for Service Pros, which is the current refinement of the method I created five years ago. This system is also taught by myself and long-time educator Greg Garrett through our newly created Pool Chemistry Training Institute, which offers chemistry instruction and certification for residential pools.
We’ve introduced some fresh approaches to pool-chemistry maintenance that are a decided departure from standard industry practice. They primarily address the two biggest chemical-related problems facing the pool industry — high CYA and low carbonate alkalinity, both of which cause corrosive water. Among the changes:
• I believe chemistry should be managed using a system of targets rather than the ranges that are recommended in standards and have been the industry’s approach for about 50 years. Standards such as ANSI/APSP/ICC-5-2011 lists minimum, ideal and maximum levels for pH, total alkalinity, total dissolved solids (TDS), calcium hardness and CYA. But the current accepted chemistry ranges allow for imbalanced water that is either scale-forming or corrosive, and seldom balanced. Shooting for precise chemistry targets is a far more sensible and simple way to achieve balanced water that makes it easy to know which conditions are on or off target and which need adjustments.
• I’ve presented additional ways to control pH, with a new buffer to help stabilize pH, and the use of aeration and turbulence to raise pH in a matter of hours with no change to alkalinity.
• This method also incorporates borate to keep pH from rising and to lower the free chlorine requirement or residual.
• It shows how to calculate chlorine residuals based on a percentage of CYA level.
Let’s examine these changes in depth.
Staying on target
Current chemical ranges are too wide or broad. Having a single target for each parameter or water condition makes it easy to know if the condition is too low, too high or on target.
For instance, current standards place the minimum pH level at 7.2 ppm, the ideal range at 7.4 to 7.6, and the maximum at 7.8. There is no instruction as to when the minimum or maximum will suffice. This creates confusion and too much room for interpretation: Everyone believes that keeping the pH anywhere between 7.2 and 7.8 will work under all conditions.
In fact, keeping all the parameters at the minimum levels will produce a Saturation Index (SI) of -0.80, which indicates very corrosive water. And using the maximum levels produces an SI of +1.10, which is severely scale-forming. (The acceptable SI range is – 0.30 to + 0.50.) So we have a standard that is so broad that you can follow the guidelines and still have problems in your pool.
Working toward specific targets eliminates the confusion and makes it easy to know what needs to be done to adjust any condition in the water.
Introducing borate
Another key concept is understanding pH buffers and how they keep the pH from changing.
Total alkalinity and CYA serve as buffers to keep pH from going down, but we traditionally haven’t had anything at our disposal to prevent the pH from rising too quickly. Borate can accomplish this. It slows down pH rise, so less acid will be needed to maintain pH. Borate is especially good for pools equipped with a chlorine generator, which can be susceptible to wider pH fluctuations.
So the combined buffer system of total alkalinity, CYA and borate keeps the pH from going up or down. This makes for a stable pH from week to week.
Borate offers other benefits. It helps prevent algae, making it an algistat (as opposed to an algicide, which kills algae). As borate helps keep algae from forming, we don’t need as much chlorine. For those following my method, with a pH of 7.5 and a CYA level of 30-50 ppm, the addition of 50 ppm borate can lower the water’s free chlorine requirement from 7.5% to 5% of CYA. That represents a 33% reduction in free chlorine. Service techs using this method have said they are buying 50% less chlorine to maintain their pools.
Aeration and turbulence as a pH control

Aeration can raise pH without affecting alkalinity. Here, an air pump is connected to an air stone, like those in aquariums. But pros can simply introduce aeration by repositioning inlets or operating waterfeatures and vanishing edges.
Aeration and turbulence can be used to raise pH with no change to alkalinity to balance pool water quickly — usually in hours or less than a day.
In this method of quickly balancing the water, acid is added to lower total alkalinity to the target of 90 ppm. To determine the total amount of acid needed, professionals can consult an acid dose app. The acid is added all at one time. This will lower pH near 6.5.
Aeration is then applied to raise pH back up to the target level of 7.5. It accomplishes this by offgassing or outgassing carbon dioxide from the water.
Professionals can introduce aeration through turbulence — aiming returns up so the water breaks the surface, as well as turning on spillways or fountains and other features that splash.
The turbulence can be turned off when pH reaches 7.5. The rate at which the carbon dioxide offgasses and the pH increases depends upon how much turbulence and aeration you can create. In an experiment, I attached a venturi injector to the output from a submersible pump, which had a flow rate of about 100 gallons per minute. This raised the pH of the pool from 6.5 to 7.5 in one hour.
If you only aim the returns up so they break the surface of the water, it could take 24 hours for the pH to reach 7.5. If you operate a negative edge or fountain or use a device that makes turbulence in the pool, it may only take hours to attain a pH of 7.5.
Once you get the pH, alkalinity, CYA and borate to the proper levels, they will remain stable and will not change.
Calculating chlorine residuals
I’ve distilled a rather complicated equation into a simpler one to show how much free chlorine is required in a pool, based on the level of CYA.
The free chlorine required for any pool is 7.5% of the CYA level in the water as a minimum or as a target. If CYA increases, this requires a compensating rise in free chlorine. My method calls for a maximum CYA of 50 ppm, for which the free chlorine required would be 3.75 ppm (50ppm x 0.075). This falls within the APSP guideline of 2.0 to 4.0 ppm, but 2.0 would not be enough to control algae.
CYA builds up quickly from trichlor use, so I generally recommend against it as a main source of chlorination. If used, this method would require that the trichlor be discontinued after the CYA level reaches 50 ppm.
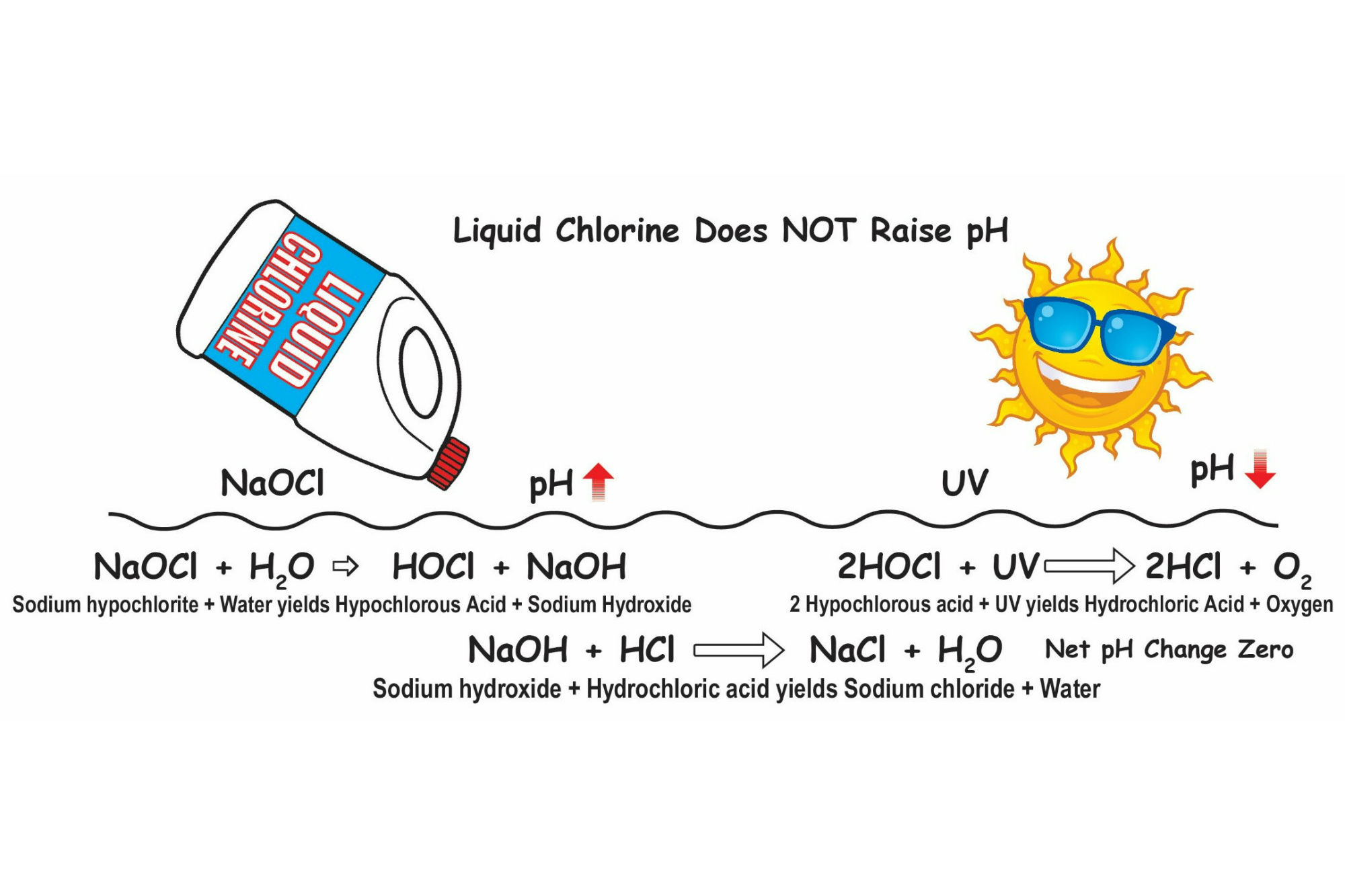
LIQUID CHLORINE DOES NOT RAISE pH: A misunderstanding about liquid chlorine, bleach and cal hypo is that they all raise the pool water pH. In actuality, the net effect on pH is zero.It is true that the pH goes up initially with the addition of these hypochlorites. This occurs as sodium hydroxide (NaOH) or calcium hydroxide (CaOH) are created along with hypochlorous acid (HOCl), the killing form of chlorine. However, as HOCl degrades through exposure to UV, bacteria or algae, it converts into hydrochloric acid (HCl), which is the non-killing form of chlorine and has the effect of reducing pH. The amount of pH-reducing hydrochloric acid created is almost equal to the pH-boosting NaOH or CaOH. So they just keep canceling each other out: The pH may go up today, but then it goes back down tomorrow.
Robert Lowry is a 45-year pool/spa-industry veteran specializing in chemistry. He has founded and co-owned two manufacturers, and has served as technical director, technology officer or consultant for numerous major industry corporations. He has written 19 pool and spa water chemistry books, all three IPSSA manuals, and published hundreds of technical articles and white papers. Last year, he and plaster expert Greg Garrett created the Pool Chemistry Training Institute. His most recent book is Pool Chemistry for Service Pros.




