Cyanuric acid, or CYA, is great at protecting chlorine from the sun’s UV rays.
But if it isn’t regularly diluted with fresh water, the chemical, a byproduct of trichlor and dichlor tablets, can quickly escalate to unwanted levels, well beyond the 30- to 50 ppm industry standard.
“A lot of times, residential pools have much more than 100 ppm of cyanuric acid,” says Robert Lowry, owner of Jasper, Ga.-based Lowry Consulting Group and author of the Independent Pool
& Spa Service Association’s Basic Training Manual.
In fact, pools using stabilized chlorine may easily exceed CYA levels of 300- to 400 ppm. That’s where the real trouble starts.
Too much CYA can trigger a number of chemistry problems, including skewed alkalinity readings, which can compromise your ability to maintain water balance; low ORP readings, which can cause unnecessary use of chemicals; and stalled chlorine, which can allow algae to sprout and spread.
What’s more, recent studies indicate that large quantities of CYA can even damage plaster, making it vitally important that service technicians understand what they’re up against, consistently monitor cyanuric acid levels, and keep those levels in check whenever possible.
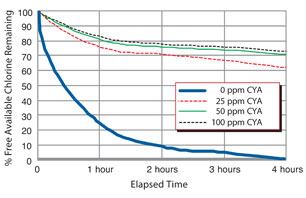
Stabilization with Cyanuric Acid
Cyanuric acid works to preserve chlorine sanitation levels in outdoor pools. But as the chart demonstrates, 25 ppm of CYA should sufficiently guard against UV rays. Notice there’s almost no difference in chlorine deactivation between 50 ppm and 100 ppm of CYA.
Alkalinity testing
The problem: High CYA levels can skew total alkalinity (TA) readings when the Saturation Index is used to determine water balance. Incorrect TA readings can lead service techs to inadvertently balance the water at a more corrosive level, with the possibility of fluctuating pH levels.
How it happens: Bicarbonate ions are key to stabilizing pH. These ions are measured through a titration test for total alkalinity. Though cyanurates are not part of this carbonate buffering system that affects pH, they will show up in a test for total alkalinity.
Thus, any reading for total alkalinity will include the effects of CYA, which does nothing to hold pH in check.
“[Testing total alkalinity], 25- to 35 percent is going to come from cyanuric acid,” Lowry says. “We have to convert that entire reading into calcium carbonate equivalence.”
The calcium carbonate equivalence is what actually stabilizes the pH, and it’s used in the Saturation Index to balance water.
To determine the adjusted TA, Lowry recommends subtracting one-third of the CYA level from the total alkalinity reading. In other words, a reading of 90 ppm cyanuric acid would account for 30 ppm of total alkalinity. Thus, for a pool with 90 ppm CYA and 100 ppm total alkalinity, the TA reading should be adjusted to the 70 ppm that actually affects the water’s pH.
It’s important to note that at pH readings of 7.5 and below, CYA has a diminished effect on total alkalinity. For example, at pH levels of 7.0, CYA accounts for less than one-quarter of total alkalinity. But with cyanuric acid levels that peak above 200 ppm at this pH, the effect is still significant because 50 ppm of a TA reading will do nothing to balance the water.
For pools with high amounts of CYA, service techs must constantly compensate for skewed alkalinity readings. Without these adjustments, the result will be unexpectedly corrosive water and unstable pH levels.
Effect of Cyanuric Acid on ORP
Higher levels of cyanuric acid generally will lead to much lower ORP readings, and adding more chlorine will not necessarily bring ORP back up. As a result, some manufacturers recommend a maximum of 20 ppm CYA to lessen the effect on ORP.
ORP readings
The problem: ORP is a common test for oxidation/sanitation in chemical controllers. But when paired with cyanuric acid, the results can be imprecise. Low ORP readings can lead to an overdose of chlorine, which is a waste of money and a potential breach of the federal Environmental Protection Agency’s chlorine limit of 5 ppm.
How it happens: ORP probes give a very accurate reading of active oxidation potential. But the test is instantaneous, which means it cannot sense the chlorine ions attached to CYA molecules. These ions, while temporarily unable to oxidize, still can be considered free chlorine because they will soon detach.
“An ORP probe takes a still picture — not a movie — of how much chlorine is free,” Lowry explains. “So there may be hundreds of connects and disconnects during that [one second] that the probe isn’t seeing.”
The bond between chlorine and CYA becomes stronger in the presence of UV rays, so this difference in readings is intensified during daylight hours, notes Ron Akin, vice president of sales at Chemtrol in Santa Barbara, Calif.
“If you look at data logs of controllers, you generally see the ORP go up at night because the bond is weakened,” he says.
One solution is to make a chart of free chlorine readings (obtained through a DPD test) and the corresponding ORP measurements. As long as the pH, total alkalinity and cyanuric acid are stable, your corresponding ORP reading should be enough sanitize the pool, regardless of the actual number.
CYA’s Effect on Bacteria Kill Time
Extremely high levels of CYA remain unstudied, but it’s clear that even at a level of 100 ppm, the bacteria kill time can be twice as long as in a pool with 50 ppm of cyanuric acid.
Because of CYA’s effect on ORP probes, Akin advises service techs to avoid stabilized disinfectants altogether on pools with
controllers. As more cyanuric acid is released into the water, ORP readings will continue to drop, causing further confusion.
He also recommends using no more than 20 ppm of cyanuric acid in pools with controllers.
“The idea is to create a ratio where you’re not locking up too much [chlorine], but not allowing it to burn up before it’s had a chance to do anything,” Akin says.
Chlorine slowdown
The problem: Unlike ammonia, CYA doesn’t actually lock up chlorine due to the weak bond. But too much CYA can slow the effectiveness of chlorine, resulting in the spread of algae and shorter filtration cycles.
How it happens: In theory, the more time chlorine ions spend on CYA molecules, the less time they have to oxidize bacteria and other contaminants.
“The more cyanuric rings you have in the water, the more time, statistically, [they’ll spend there],” says Ellen Meyer, technology manager at Arch Chemicals’ Water Products in Charleston, Tenn.
And lower concentrations of chlorine appear to be more at risk, according to studies (see chart above). For instance, the effect of CYA on a pool with 0.5 ppm of chlorine is exponentially greater than for a pool with 1 ppm of chlorine.
It’s still unclear to what extent CYA affects the performance of chlorine. But many state health departments have set a maximum level for cyanuric acid at 100 ppm for commercial pools to prevent interference with chlorine.
Problems in plaster
The problem: Studies by Arch Chemicals and the National Pool Industry Research Center on the effects of cyanuric acid on plaster found extensive degradation, as well as large amounts of algae, in the high-CYA (beyond 100 ppm) test pools.
How it happens: With high levels of CYA, the acid actually leaves the water and attaches itself to the plaster.
In 2004, Arch Chemicals studied this phenomenon by testing pools with cyanuric levels of 0, 25, 50, 100, 250 and 500 ppm. Under electron microscopy, researchers first observed the effect on plaster in water with 100 ppm of the chemical, Meyer says. The tests were performed in lab tanks and pools, with the same results: The higher the levels of CYA, the greater the deterioration of the plaster.
“The cyanuric acid was no longer in solution,” Meyer says. “It was on the plaster surface, having some kind of effect.”Meanwhile, the impact on water with 250- and 500 ppm CYA was more clearly visible to the naked eye.
Beyond observations in a lab, plasterers have noticed this effect, too.
“I’ve seen hundreds of pools in the last year [with plaster problems],” says Kevin Kostka, who performs pool start-ups and quality control for Alan Smith Pool Plastering in Orange, Calif. “This cyanuric [issue] comes up every single time. It’s obvious when you see enough of them.”
The solution: dilution
Dilution is widely considered the most logical approach to lowering CYA levels.
Phil Sharp, owner of River City Pool Service in San Antonio, says he’d rather drain and refill a pool entirely than waste even more money on chemicals to rectify a water chemistry imbalance.
Sharp tests his pools every other week for CYA to ensure the levels remain under control.
“You’ve got to test for cyanuric acid, no matter what you’re using,” he says. “If your ranges are out of control, it’ll create huge problems.”
Service techs could always switch to using unstabilized disinfectants, but for many, this is rarely an ideal solution.
“When you use dichlor or trichlor, you’re constantly [putting in] cyanuric acid. … But a lot of people will use the dry chemicals because they have a longer storage life,” Akin says.
Techs may prefer trichlor for its ease of use. Also, without using stabilized sanitizers, cyanuric acid must be added eventually because rain and splash-out would lower a pool’s CYA levels over time.
But if you do use stabilized chlorine, testing for CYA is critical.